The most commonly used active electrode is the copper electrode. Depending on the type of electrode used and the method of protecting the weld from oxidation arc welding can be.

Solved Be Sure To Answer All Parts Consider The Voltaic Chegg Com
Any material that conducts electricity.
. Zn s Zn2aq 2e- in Zn metal Cu s. The electrode sheet was dried at 180C for 8 h and compressed to control the electrode density so that the porosity of the electrode was 0305. The metal electrodes are immersed in electrolyte solutions.
DeltaG-nFE_cell Write the equation that relates cell potential to the. Second it demonstrates the electrode binder as an enabling technology for Si-based electrode. The electrochemical performance of lithium iron phosphate LiFePO 4 electrodes has been studied to find the optimum content of inactive materials carbon black polyvinylidene difluoride PVDF polymer binder and to better understand electrode performance with variation in electrode composition.
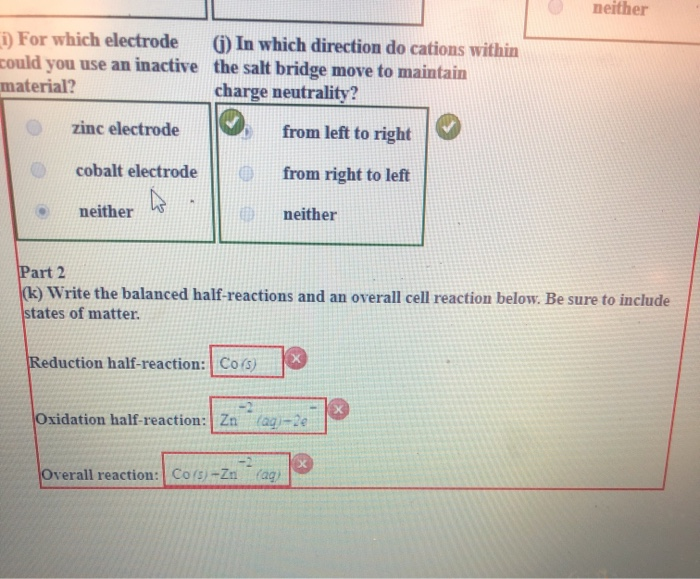
An electrosurgical unit ESU consists of a generator and a handpiece with one or more electrodes. An example of electrolysis using reactive electrode is the electrolysis of copper II sulfate using copper electrodes for the cathode and anode. Cobalt electrode neither neither i For which electrode could j In which direction do cations within the you use an inactive material.
D At which electrode are electrons generated. After calendering the electrode was vacuum-dried again at 180C for 8 h. Apperatus that change direct current into alternating current.
Inactive electrodes are usually unreactive substances such as graphite or platinum. Copper is second only to silver in terms of bulk electrical conductivity. A major amount of current flow is monitored for the conductive-carbon.
It makes up. What is the difference between an active and an inactive electrode. At the anode oxidation takes place and at the cathode reduction takes place.
Trade-offs between inactive material content and electrochemical. The Molecular Nature of Matter and Change 5th Edition Edit edition Solutions for Chapter 21 Problem 25P. The electrodes are considered to be conductors of electric current which at the same time serves as additional material in arc welding.
The active electrode is called active because it actively participates in the chemical reaction. An active electrode is defined as a metal that is used in electrochemical cells. Zinc electrode cobalt electrode neither zinc electrode zinc electrode.
Active electrodes are used in electroplating. An electrochemical cell needs two electrodes namely anode and cathode. A Current flow as a function of time during a constant potential step at 495 vs.
Es do not depend on amounts of material. These electrodes can be oxidized or reduced. Oppisite pole from the active electrode.
7 From Levels wallpaper. Copper is a common base metal for electrical contact. LiLi for 24 h using an Al electrode a binderAl electrode and a conductive-carbonbinderAl electrode to systematically investigate the contribution of each inactive material.
An produces more heat than visible lights. Another definition would be that an unreactive metal rod placed in the solution can be called an inert electrode. An active electrode is an active component in its half cell and is a reactant or product in the overall reaction.
Infrared lights have a longer wavelengthpenerates more deeplyhas less energy. Electrical Potential and the Voltaic CellWhen the switch is closed and no reaction is occurring each half-cell is in an equilibrium state. The chapter details bottom-up design principles for functional.
Table I lists the final porosity and density of the active material for each electrode. For Which Electrode Would You Use an Inactive Material. An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit eg.
Copper has better strength than silver but offers inferior oxidation resistance. Inert electrode is an electrode that does not participate in the chemical reaction. Inert electrodes are used in electrolysis.
F Which electrode increases in mass during cell operation. An electrode which has no ions in common with the solution may be called an inert electrode. Active electrode are part of the half cell redox reaction inactive electrodes are not.
7 Levels of Classification From Largest to Smallest. This is because copper II ions are preferentially discharged as copper is lower than hydrogen in the. Why are inactive electrodes used.
The device is controlled using a switch on the handpiece or a foot switch. Salt bridge move to maintain charge neutrality. Zns Zn 2 aq 2 e Copper electrode cathode.
What is the difference between an active and an inactive electrode. Each half-cell is connected by a salt bridge which allows for the free transport of ionic species between the two cells. As these waveforms change so do the corresponding tissue effects.
Cu 2 aq 2 e Cus The cells are constructed in separate beakers. For example in the electrolysis of water H2O acidified with sulfuric acid H2 SO4 using platinum electrodes platinum is an inert electrode. Also it is used in electroplating.
The reactants in the electrolyte get oxidizedreduced at the surface of the inert electrode. Electrodes are essential parts of batteries that can consist of a variety of materials depending on the type of battery. A semiconductor an electrolyte a vacuum or air.
First it elucidates the effects of active and inactive phases of the Si and SiO 2 for the battery performance and the effects of necessary carbon coating and composite with Si materials. Some of the most prominent alloys and materials used as electrode materials are copper graphite titanium brass silver and platinum. At the cathode copper II ions will be deposited hence a brown solid is formed at the cathode.
By Bio_Kenna66 19 Apr 2022 Post a Comment G Suggest a solution for the anode electrolyte. Active electrode is the electrode that actively takes part in the chemical reaction of the electrochemical cell. When we use inert electrodes such as platinum it wont get oxidized or reduced.
The electrophore invented by Johan Wilcke was an early version of an electrode used to study static electricity. Electrosurgical generators can produce a variety of electrical waveforms. Today in practice we usually use bare or coated metal electrodes.
Write the equation that relates free energy to cell potential.
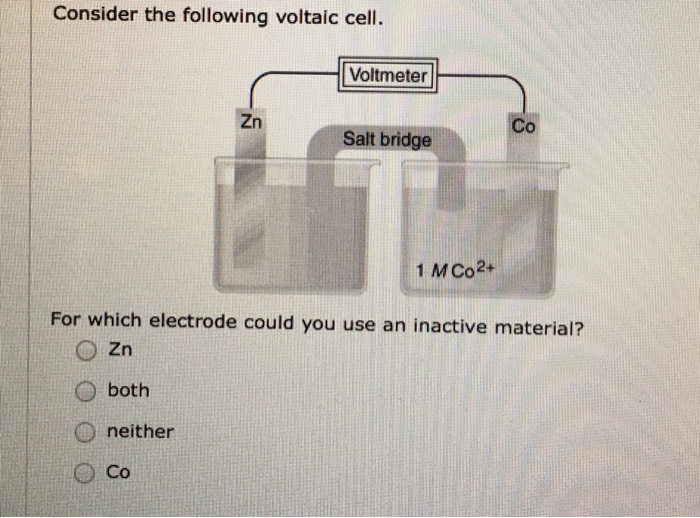
Solved Consider The Following Voltaic Cell For Which Chegg Com

Solved This Window Shows Your Responses And What Was Marked Chegg Com

Answered Consider The Voltaic Cell Below Bartleby
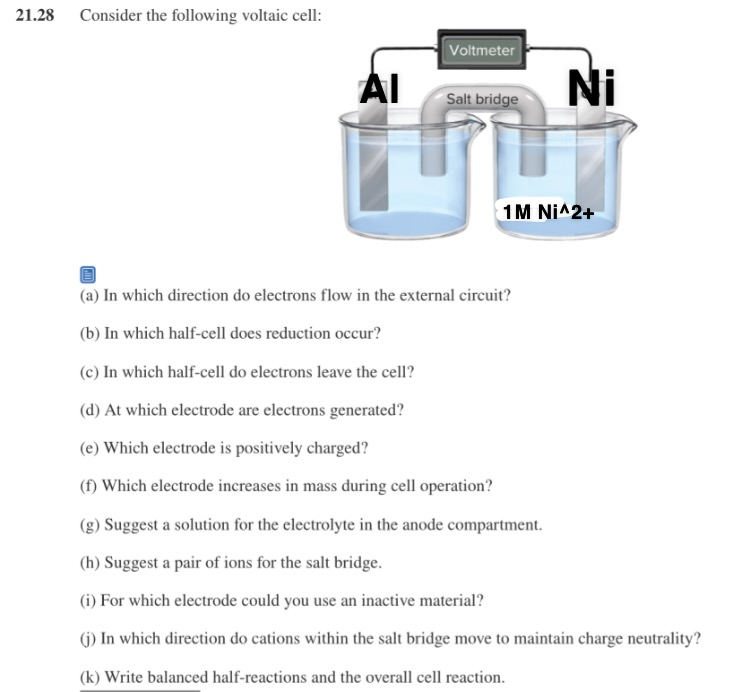
Solved 21 28 Consider The Following Voltaic Cell Voltmeter Chegg Com
0 Comments